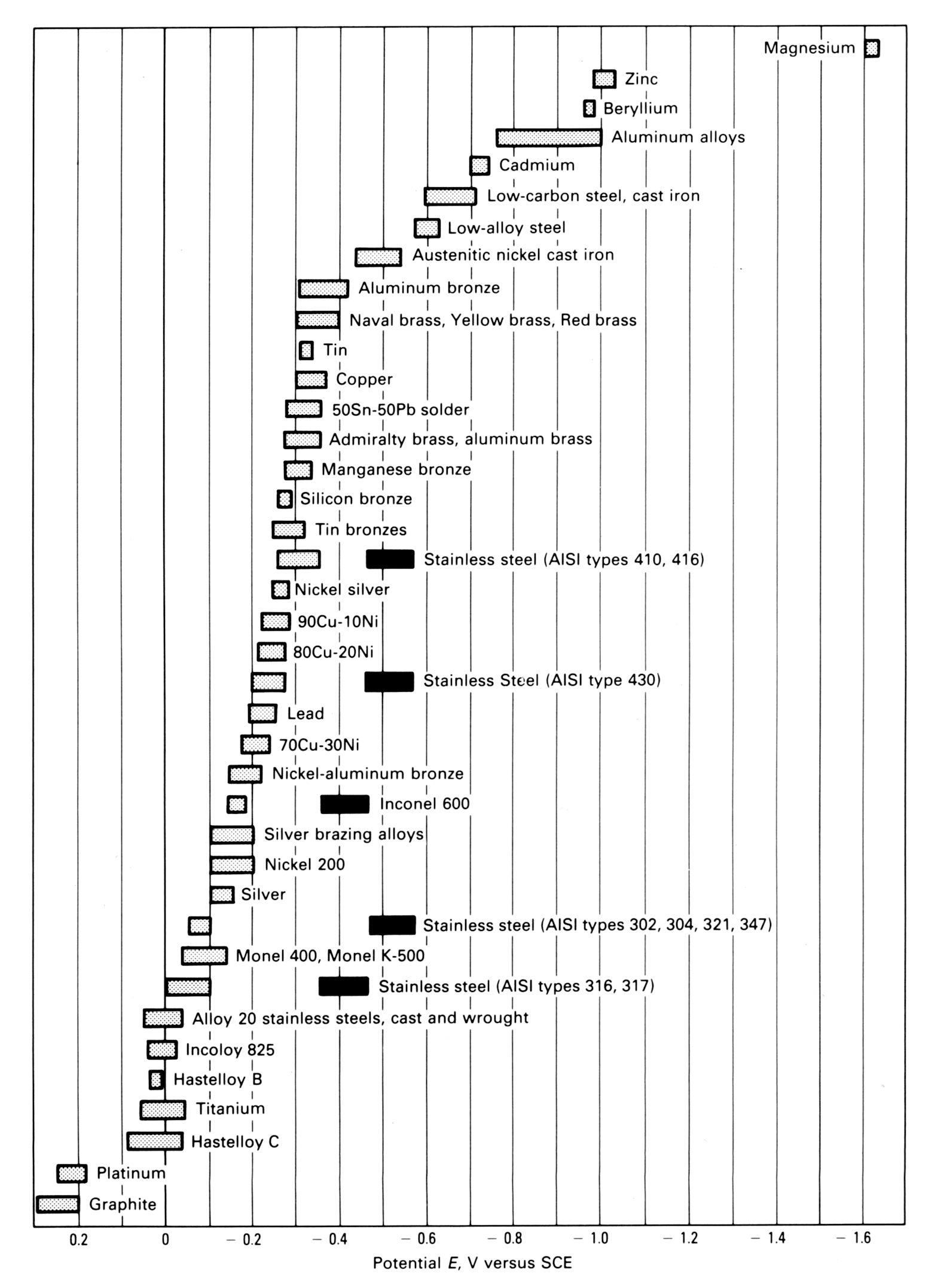
Galvanic Reaction Chart
Galvanic Reaction Chart - Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with an electrolyte. There are three conditions that must. How to use galvanic in a sentence. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. It achieves this by harnessing the energy produced by the redox. Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte.
Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with an electrolyte. A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same. The meaning of galvanic is of, relating to, or producing a direct current of electricity. There are two primary types of galvanic cells that cause corrosion: This phenomenon results from the.
There are three conditions that must. A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same. It achieves this by harnessing the energy produced by the redox. The meaning of galvanic is of, relating to, or producing a direct current of electricity. A galvanic.
A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with.
Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with an electrolyte. There are two primary types of galvanic cells that cause corrosion: Galvanic corrosion is a type of electrochemical corrosion that occurs when two dissimilar metals are in electrical contact with each other and are simultaneously exposed to an electrolyte,..
How to use galvanic in a sentence. Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with an electrolyte. It achieves this by harnessing the energy produced by the redox. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes.
Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is.
The meaning of galvanic is of, relating to, or producing a direct current of electricity. Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte. How to use galvanic in a sentence. Galvanic corrosion, also known as bimetallic corrosion or dissimilar metal.
A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. There are three conditions that must. This phenomenon results from the. Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte. Galvanic corrosion, also known as.
Galvanic corrosion, or bimetallic corrosion, occurs when one metal corrodes faster than another when both are in contact with an electrolyte. There are two primary types of galvanic cells that cause corrosion: Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte..
Galvanic Reaction Chart - This phenomenon results from the. Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte. Galvanic corrosion is a type of electrochemical corrosion that occurs when two dissimilar metals are in electrical contact with each other and are simultaneously exposed to an electrolyte,. A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. There are three conditions that must. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. The meaning of galvanic is of, relating to, or producing a direct current of electricity. There are two primary types of galvanic cells that cause corrosion:
Galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in contact. How to use galvanic in a sentence. There are two primary types of galvanic cells that cause corrosion: A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same. This phenomenon results from the.
Galvanic Corrosion (Also Called Bimetallic Corrosion Or Dissimilar Metal Corrosion) Is An Electrochemical Process In Which One Metal Corrodes Preferentially When It Is In Electrical.
This phenomenon results from the. There are three conditions that must. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Galvanic corrosion is one of the most significant and challenging forms of metal degradation, occurring when two dissimilar metals come into contact in the presence of an electrolyte.
Galvanic Corrosion, Or Bimetallic Corrosion, Occurs When One Metal Corrodes Faster Than Another When Both Are In Contact With An Electrolyte.
It achieves this by harnessing the energy produced by the redox. The meaning of galvanic is of, relating to, or producing a direct current of electricity. Galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in contact. There are two primary types of galvanic cells that cause corrosion:
How To Use Galvanic In A Sentence.
Galvanic corrosion is a type of electrochemical corrosion that occurs when two dissimilar metals are in electrical contact with each other and are simultaneously exposed to an electrolyte,. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A galvanic reaction (also known as galvanic corrosion) occurs when two metals with different electrochemical potentials are in direct electrical contact and immersed in the same.

![Galvanic Corrosion [with Chart] EngineerExcel](https://i2.wp.com/engineerexcel.com/wp-content/uploads/2023/03/galvanic-compatibility-1024x684.jpeg)